Διάλεξη
Τρίτη 18 Σεπτεμβρίου 2018
- Ομιλητής
- Paweł Rodziewicz
- Ίδρυμα
- Institute of Chemistry, University of Bialystok, Poland
- Τίτλος
- Sulfur mustard molecule on ZnO surface: structural diversity from first principles calculations
- Χώρος
- Αίθουσα A2 (Α115-Α117) του κτιρίου Επιστήμης Υπολογιστών
- Ώρα
- 12:00
- Γλώσσα
- Αγγλικά
- Περίληψη
-
Chemical warfare agents (CWAs) have been used for the first time in a massive way during the World War I. [1] One of the most dangerous CWAs were skin damaging agents like sulfur mustard (bis(2-chloroethyl)sulfide), used by German Forces during the battle of Ypres on 12 July 1917. Even though, CWAs were an extremely dangerous weapon, neither Allied Powers nor Axis used them on the large scale in the World War II. The postwar arsenals of the chemical weapons were partially kept during the Cold War to act as a deterrent. In 1997 the international treaty, known as Convention on the Prohibition of the Development, Production, Stockpiling and Use of Chemical Weapons and on their Destruction, entered into force.
The sulfur mustard (SM) is one of the most common CWAs, and due to its easy sythesis, is still used as a chemical weapon, for example in Yemen or Iraq. [1] There is still a high risk of the usage of SM by terrorists so the efficient and fast methods of its neutralisation should be developed. Ideally, the neutralization of SM should lead fast to 100% non-toxic products and the substrate itself should remain non-toxic and keep its high surface area. Additional condition for efficient decontamination is the presence of water on the surface which takes a crucial role in the SM hydrolysis. [2, 3] All of these criteria were fulfilled by zinc oxide nanorods, used successfully as reactive sorbent for the detoxification of SM at room temperature. [2]
The small size of the SM molecule and available theoretical models of the ZnO surface make the adsorption of SM on ZnO an ideal target for the studies utilizing the state-of-the-art theoretical methods. Our main aim was to describe the energetics of the SM molecule on the ZnO surface, in particular to define its global and local minima for the clean and water covered ZnO surfaces. We focused on the structural properties of the adsorbed SM molecule with the special focus on the subtle interplay between SM–ZnO and SM–water interactions. Our theoretical studies, namely combined static DFT calculations and Car-Parrinello molecular dynamics simulations, shed light on the role of the intra- and intermolecular hydrogen bonds in the neutralization process of the SM molecule on the ZnO surface.
- L. Szinicz. History of chemical and biological warfare agents. Toxicology, 214:167, 2005.
- G. K. Prasad, T. H. Mahato, B. Singh, K. Ganesan, P. Pandey, and K. Sehhar. Detoxification reactions of sulphur mustard on the surface of zinc oxide nanosized rods. J. Hazard. Mater., 149:460, 2007.
- G. K. Prasad, P. V. R. K. Ramacharyulu, B. Singh, K.Batra, A. R. Srivastava, K. Ganesan, and R. Vijayaraghavan. Sun light assisted photocatalytic decontamination of sulfur mustard using ZnO nanoparticles. J. Mol. Catal. A: Chem., 349:55, 2011.
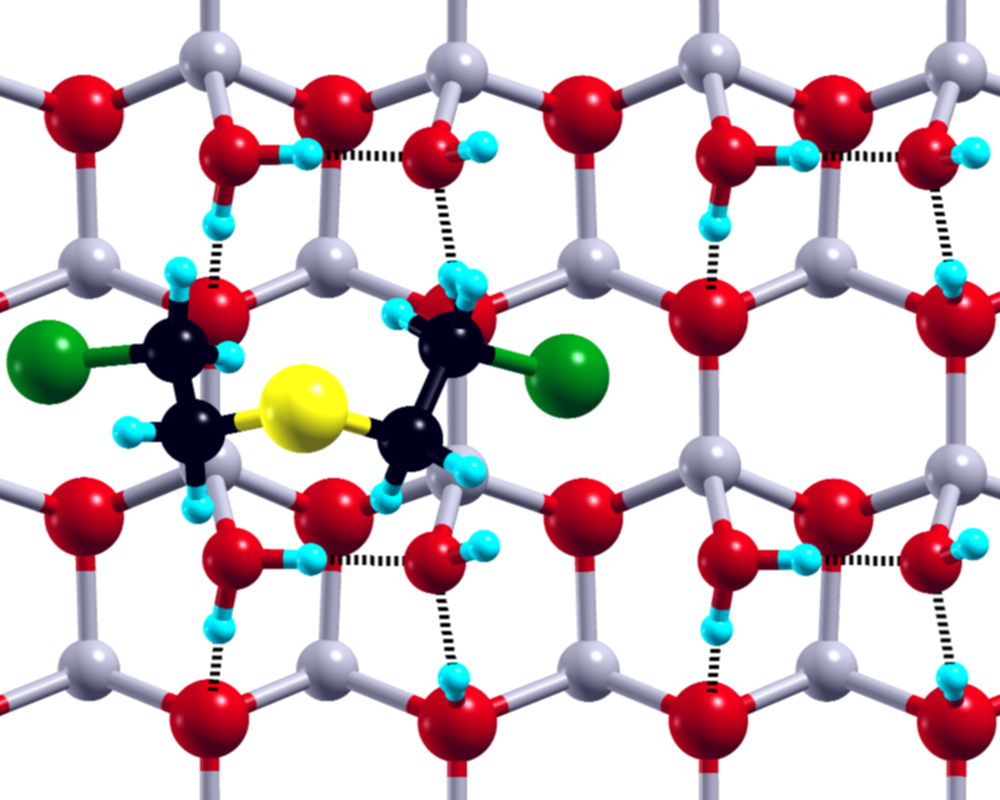
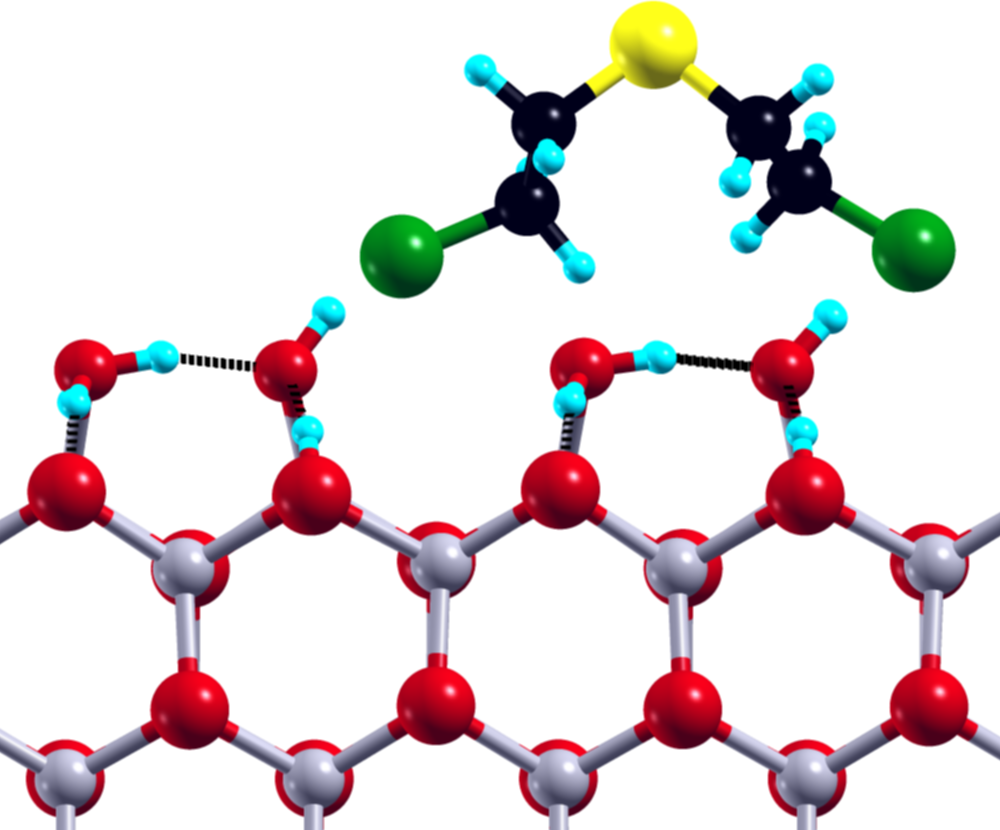
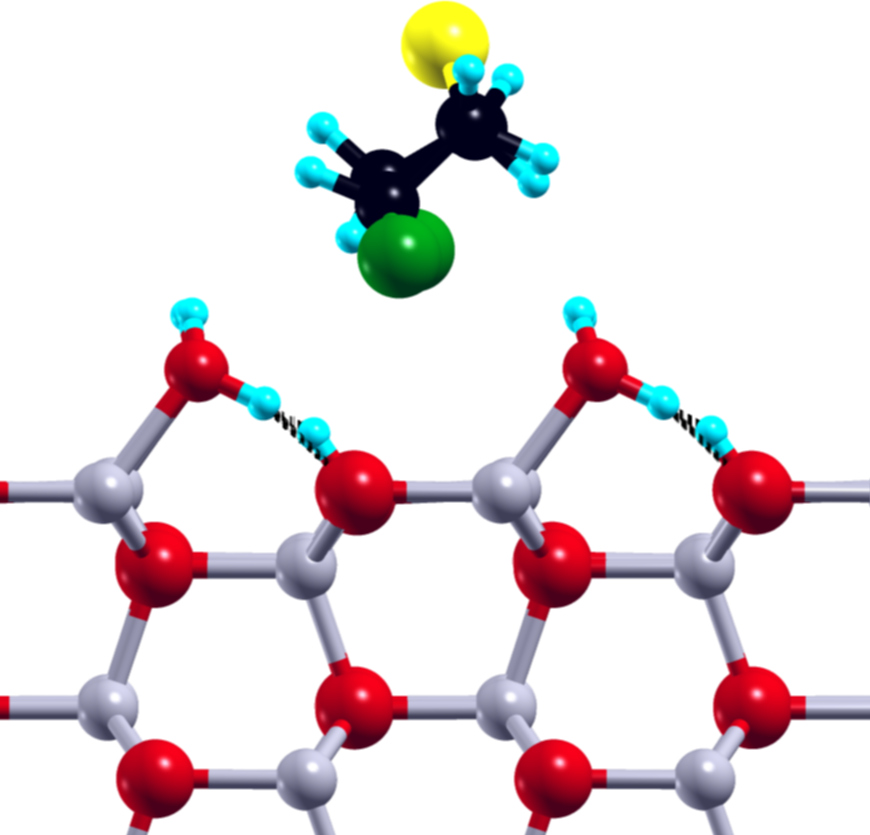
1: SM@ZnO-W:1 Ebind = -17.20 kcal/mol Einter = -18.88 kcal/mol
Figure 1: Selected SM structure, with the respective binding and interaction energies, after adsorption at the ZnO surface, plane of view: XY (left), XZ (middle), ZY (right).
For forthcoming colloquia, please see: http://www.materials.uoc.gr/el/colloquia